What to consider in autoinjector development projects
April 23 2025
Factors such as drug properties, user needs, and manufacturing must be considered in autoinjector development.
As the leading autoinjector solutions provider, we understand the challenges in navigating the most suitable option amidst various platform or bespoke selections. When customers approach us with their needs, multiple factors are taken into consideration, such as the formulation viscosity, the intended dosage volume, and market readiness, since all this impacts how the autoinjector is developed.
At SHL Medical, we tailor our offerings to meet specific needs of our pharma partners. Below is a graph that shows how varying formulation viscosities and fill volumes could affect the choice of drug delivery offerings we recommend.
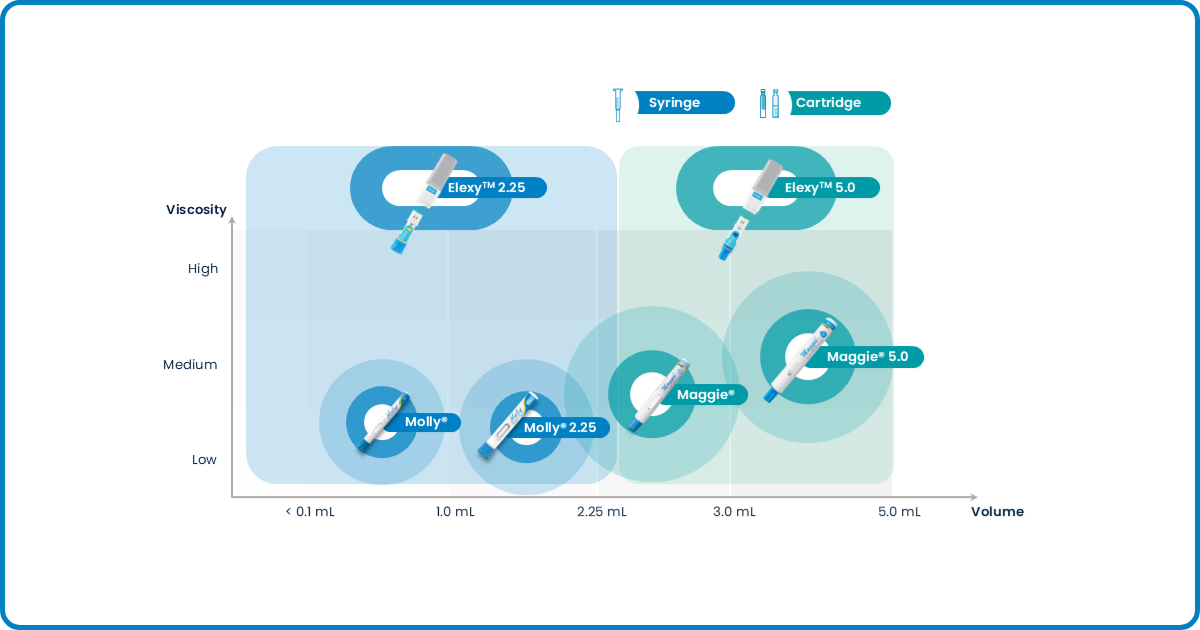 Figure 1: An overview of SHL Medical's device solutions to accommodate various biologic formulations and primary containers
Figure 1: An overview of SHL Medical's device solutions to accommodate various biologic formulations and primary containers
To help SHL Medical offer the right solution to a pharma customer, we first gather as much information as possible to help speed up the process. Is the molecule an originator biologic or a biosimilar? At which phase is the biological medicine development project? What primary container is used for the biologic? How viscous is the biologic formulation? These are just a few questions we need answers to. Let’s dive deep into some of the questions.
What primary container is used for the biologic?
The choice of primary container—whether a pre-filled syringe (PFS) with staked needle or a cartridge—will heavily drive the autoinjector’s design and technology. Considered secondary packaging, SHL Medical’s autoinjector components are developed around the primary packaging (containers in direct contact with the medication) with the goal of successfully delivering an accurate dose to the patient.
To support autoinjector testing and development, pharma and biotech partners must provide formulation samples in their designated primary containers, along with detailed specifications of the formulation characteristics. These insights enable our team to assess drug-device compatibility, optimize performance, and advance product development efficiently.
What is the fill volume and the required dosage for the biologic?
How much of the biologic does the patient require for effective treatment? To develop the right drug delivery device, we need information on the dosage volume and primary container capacity. For example, a 0.5 mL biologic formulation is typically housed in a 1.0 mL prefilled syringe. In recent years, market demand for higher-volume subcutaneous therapies—exceeding 3.0 mL—has grown, driven by the rise of complex biologics that require higher molecule concentrations to elicit a clinical effect.
What is the viscosity of the biologic formulation?
Like the dosage volume, the viscosity of the biologic will also affect the autoinjector’s spring design, as viscous formulations tend to require greater force to push through a thin-walled needle.
If you require a drug delivery device for high-viscosity formulations, SHL Medical will develop a robust spring mechanism to ensure consistent and reliable delivery. Our team works closely with pharma and biotech partners to optimize injection performance, balancing injection force requirements with patient comfort and usability.
What is the intended injection time?
Factors such as formulation volume, viscosity, and the needle gauge all impact injection speed. Providing a clear injection time requirement allows SHL Medical’s experts to fine-tune the autoinjector’s spring mechanism and component design to meet the intended dosage delivery and target injection time.
For optimal user experience, the injection time should be kept within a reasonable duration, typically no longer than 50 seconds. Our team balances injection force and timing to enhance usability, minimize patient discomfort, and ensure efficient and controlled medication administration.
Who are the target users?
At SHL Medical, patient needs are at the core of our autoinjector designs. Understanding the target users—including their age, disease state, and habits—is essential to developing a device that ensures safety and ease of use.
Factors such as dexterity limitations, vision impairment, or mobility challenges can influence design elements of the autoinjector such as grip ergonomics, activation force, and visual or audible feedback. Additionally, knowing when and where patients typically self-inject will help determine features like portability, ease of handling, and autoinjector kit configuration. By considering these aspects, SHL Medical ensures that our autoinjectors are designed not just for functionality but for real-world patient experiences.
Platform or Bespoke?
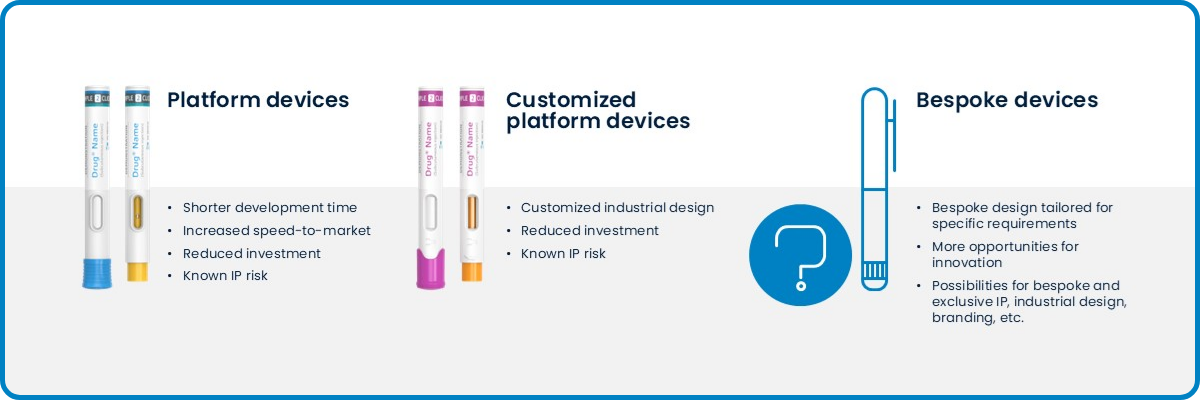
At SHL Medical, we offer two development approaches: platform or bespoke projects. Customers have the choice of going for a fully customized autoinjector tailored to their specific needs or building upon an already-established platform solution.
A bespoke project involves developing a completely new autoinjector from the ground up. The process begins with in-depth design discussions, where our team works closely with customers to define injection parameters and user needs, as well as drive usability testing for the targeted patient group. Since each component is designed and validated from scratch, the design and development timeline is longer before moving into engineering and production.
Platform projects allow for a more mature starting point, such as having a predefined set of autoinjector components. There will typically be less investment in assembly or molding process development as the required tools or infrastructure have already been developed. It is important to know that platform projects are not off-the-shelf products. Each platform-based autoinjector project still undergoes a certain degree of device customization to align with formulation and patient requirements.
At SHL Medical, we offer customized platform solutions, allowing for a slightly higher level of modification compared to the standard platform approach. This could include adjustments in several colored components for branding or usability purposes. It is worth to note that color changes in components involve more than just aesthetics. The development process entails careful selections of plastic resins, material testing, and trial runs in plastic injection molding.
By providing both bespoke and platform options, SHL Medical enables customers to choose a solution that balances customization, cost, and development speed based on their needs.
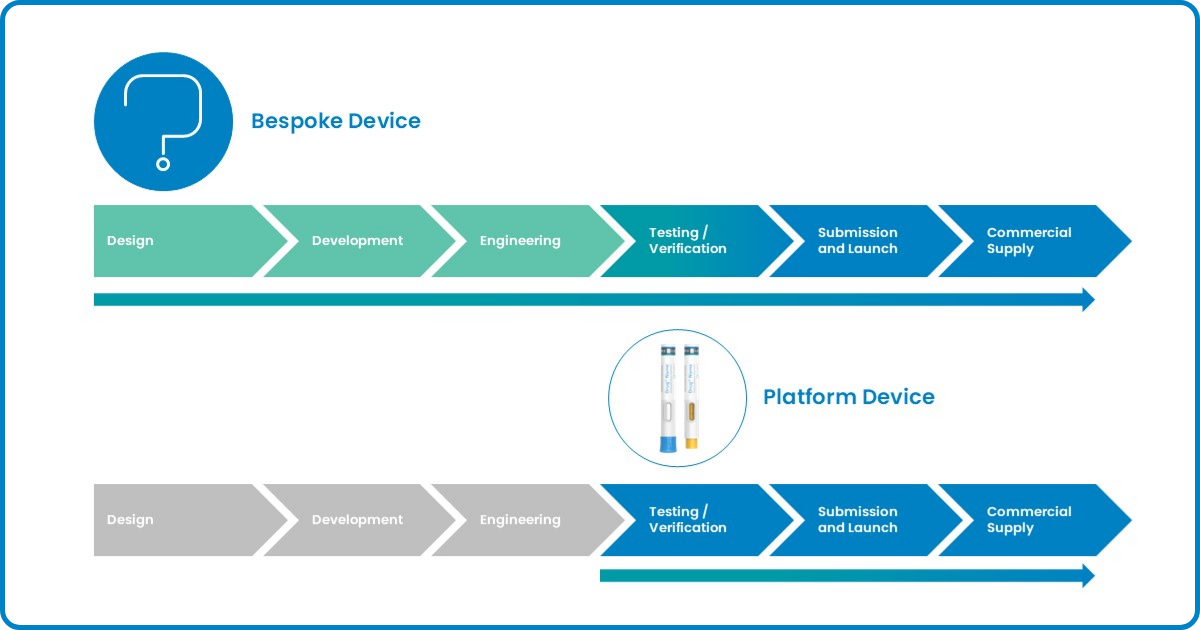
Here is a timeline comparison illustrating the different development durations for bespoke and platform projects.
Figure 2: Development timelines for bespoke and platform projects at SHL Medical
As mentioned in the previous sections, every project at SHL Medical—whether bespoke or platform—follows a structured development process. Each request from our partners initiates a project that must go through key stages, including testing, verification, documentation, and various engineering activities before reaching commercial supply. Regardless of the development pathway, the resultant combination product must undergo approval from regulatory authorities before commercial distribution.
Here are the different benefits of choosing a bespoke or platform solution:
Figure 3: Different benefits of choosing a bespoke or platform solution
If you would like to find out more about what exactly defines an autoinjector and how autoinjectors came into form, check out these two blogs:
● Exploring the basics of autoinjectors
● What did humans use before the invention of autoinjectors?