Winning combinations
Since the launch of our first injector in 1996, around four dozen combination products using devices designed and developed by SHL have been approved around the world. These winning combinations are delivered to patients by leading pharmaceutical brands in therapeutic areas such as multiple sclerosis, rheumatoid arthritis, migraine, type 2 diabetes, and hypercholesterolemia.
Owning over 20 years of experience in the medical device industry, SHL has grown into a global organization that boasts quite the formidable customer base. SHL has helped shape industry standards for the usability of autoinjectors with innovative designs and cutting-edge manufacturing processes. From bespoke projects to preconfigured devices, all our offerings are developed in close partnership with biopharmaceutical clients to ensure that their innovative, life-changing drugs are delivered to patients safely and efficiently.
Since the launch of our first injector in 1996, around four dozen combination products using devices designed and developed by SHL have been approved around the world. These winning combinations are delivered to patients by leading pharmaceutical brands in therapeutic areas such as multiple sclerosis, rheumatoid arthritis, migraine, type 2 diabetes, and hypercholesterolemia.
Owning over 20 years of experience in the medical device industry, SHL has grown into a global organization that boasts quite the formidable customer base. SHL has helped shape industry standards for the usability of autoinjectors with innovative designs and cutting-edge manufacturing processes.
From bespoke projects to preconfigured devices, all our offerings are developed in close partnership with biopharmaceutical clients to ensure that their innovative, life-changing drugs are delivered to patients safely and efficiently.
Quick to Market™ services: fully integrated competencies for optimized development
The Molly® modular platform technology offers not just flexibility in design and manufacturing, but also optimization in product development.
In 2017, a leading global healthcare company kicked off a project based on the second-generation Molly platform – chosen to fulfill the customer’s need for its own platform autoinjector for unique branding, high-volume production, as well as faster development across different combination product projects. SHL rose to the challenge with end-to-end development capabilities and fully integrated services supporting clinical and commercial delivery. The modular approach to platform development allowed us to leverage existing methodologies, documentation, as well as production infrastructures to support faster development. In just a year after the combination product received its first approval, the autoinjector – featuring the same industrial design with different color combinations – was approved as a second combination product in the US and in Europe.
Both these combination product projects were supported by SHL’s Quick to Market™ services – a program featuring a range of in-house competencies and services in support of speeding up marketing lead time. As a result of the streamlined processes, every important milestone of the second combination product project was achieved within around a year of each other. The project also advanced from US FDA approval to its first commercial delivery in under a month. That same year, the autoinjector also went through the EU Medical Device Regulation Notified Body opinion with a positive outcome.
The Molly® modular platform technology offers not just flexibility in design and manufacturing, but also optimization in product development.
In 2017, a leading global healthcare company kicked off a project based on the second-generation Molly platform – chosen to fulfill the customer’s need for its own platform autoinjector for unique branding, high-volume production, as well as faster development across different combination product projects. SHL rose to the challenge with end-to-end development capabilities and fully integrated services supporting clinical and commercial delivery. The modular approach to platform development allowed us to leverage existing methodologies, documentation, as well as production infrastructures to support faster development. In just a year after the combination product received its first approval, the autoinjector – featuring the same industrial design with different color combinations – was approved as a second combination product in the US and in Europe.
Both these combination product projects were supported by SHL’s Quick to Market™ services – a program featuring a range of in-house competencies and services in support of speeding up marketing lead time. As a result of the streamlined processes, every important milestone of the second combination product project was achieved within around a year of each other. The project also advanced from US FDA approval to its first commercial delivery in under a month. That same year, the autoinjector also went through the EU Medical Device Regulation Notified Body opinion with a positive outcome.
Molly® and Molly® 2.25: SHL Medical’s modular platform autoinjector
With more platform products emerging in the autoinjector space, SHL sought to redefine how platform device technologies can disrupt the market. Further refined to offer the advantages of platform products while offering flexibility in the device’s design, development, and production – the Molly modular platform was born.
This new generation of Molly was put to test with a leading pharma partner of SHL needing a customizable device offering for two of its biologics – a molecule for atopic disorders and another for hyperlipidemia. The device had to address the customer’s industrial design and branding preferences, as well as the primary container of the biologics in question. Building upon the successes of its predecessor, the Molly modular platform technology was able to fulfill the projects’ specifications, resulting in customized device families featuring distinct industrial designs that are conformant to the primary container. Of important note, the Molly 2.25 variant supported the development and commercialization of the world’s first autoinjector in the higher volume range (≥2.0 mL).
This evolution of Molly as a flexible solution to SHL’s pharmaceutical partners will continue with modular expansions that add value to Molly’s ecosystem and increasing commercial successes around the world.
With more platform products emerging in the autoinjector space, SHL sought to redefine how platform device technologies can disrupt the market. Further refined to offer the advantages of platform products while offering flexibility in the device’s design, development, and production – the Molly modular platform was born.
This new generation of Molly was put to test with a leading pharma partner of SHL needing a customizable device offering for two of its biologics – a molecule for atopic disorders and another for hyperlipidemia. The device had to address the customer’s industrial design and branding preferences, as well as the primary container of the biologics in question. Building upon the successes of its predecessor, the Molly modular platform technology was able to fulfill the projects’ specifications, resulting in customized device families featuring distinct industrial designs that are conformant to the primary container. Of important note, the Molly 2.25 variant supported the development and commercialization of the world’s first autoinjector in the higher volume range (≥2.0 mL).
This evolution of Molly as a flexible solution to SHL’s pharmaceutical partners will continue with modular expansions that add value to Molly’s ecosystem and increasing commercial successes around the world.
Molly®: SHL Medical’s first preconfigured autoinjector
The establishment of the Molly platform signifies SHL’s commitment to further addressing the varying needs of our customers and their patients. Introduced in 2010, Molly is SHL’s first preconfigured autoinjector designed to help pharmaceutical companies reduce initial investments and expedite development timelines.
Molly features just two simple steps: uncap and inject. As a platform device technology, Molly is supported by SHL’s in-house tooling, assembly, testing, and final assembly infrastructure that is designed to support a fully streamlined product development. Just a year after its launch, Molly went on to win the 2011 Good Design Award, signifying its embodiment of a society-leading good design.
The commonality of Molly’s platform infrastructures has allowed SHL to undertake various overlapping device projects. In 2016 alone, this preconfigured offering has enabled the commercial launch of at least three combination products indicated for inflammatory and autoimmune diseases, as well as migraine. With the Molly technology’s vertically integrated development model, SHL also fulfilled the final assembly, labeling, and packaging requirements of the migraine device. These Quick to Market™ services led the migraine device project to advance from device development to clinical delivery in less than nine months.
The establishment of the Molly platform signifies SHL’s commitment to further addressing the varying needs of our customers and their patients. Introduced in 2010, Molly is SHL’s first preconfigured autoinjector designed to help pharmaceutical companies reduce initial investments and expedite development timelines.
Molly features just two simple steps: uncap and inject. As a platform device technology, Molly is supported by SHL’s in-house tooling, assembly, testing, and final assembly infrastructure that is designed to support a fully streamlined product development. Just a year after its launch, Molly went on to win the 2011 Good Design Award, signifying its embodiment of a society-leading good design.
The commonality of Molly’s platform infrastructures has allowed SHL to undertake various overlapping device projects. In 2016 alone, this preconfigured offering has enabled the commercial launch of at least three combination products indicated for inflammatory and autoimmune diseases, as well as migraine. With the Molly technology’s vertically integrated development model, SHL also fulfilled the final assembly, labeling, and packaging requirements of the migraine device. These Quick to Market™ services led the migraine device project to advance from device development to clinical delivery in less than nine months.
PPI®: High-precision autoinjector for extremely viscous formulations
The development of the Precision Pen Injector (PPI) is a story of uncompromising dedication to meeting industry challenges.
A Swedish company specializing in solutions for aesthetics and corrective treatment sought to introduce an autoinjector for its skin booster – a highly viscous hyaluronic acid formulation of several hundred centipoise. The company needed a device that could deliver small and very precise doses of this treatment while addressing the challenges the filler market did not have at the time, such as drooling and overdosing.
To meet these challenges, our design engineers created the Rotaject®, a patented clock spring technology that made it possible for a mechanically driven injector to deliver small (10 μL) and consistent dosages of highly viscous formulations for up to 200 times from a 2.25 mL pre-filled syringe. Working with various study groups, we developed a user-friendly device that maintained a balance between its shape and weight versus its mechanical properties. The result was the PPI – a sleek and aesthetically pleasing pen injector that went on to win the prestigious Red Dot Design Award for product design in 2009.
The development of the Precision Pen Injector (PPI) is a story of uncompromising dedication to meeting industry challenges.
A Swedish company specializing in solutions for aesthetics and corrective treatment sought to introduce an auto-injector for its skin booster – a highly viscous hyaluronic acid formulation of several hundred centipoise. The company needed a device that could deliver small and very precise doses of this treatment while addressing the challenges the filler market did not have at the time, such as drooling and overdosing.
To meet these challenges, our design engineers created the Rotaject®, a patented clock spring technology that made it possible for a mechanically driven injector to deliver small (10 μL) and consistent dosages of highly viscous formulations for up to 200 times from a 2.25 mL pre-filled syringe. Working with various study groups, we developed a user-friendly device that maintained a balance between its shape and weight versus its mechanical properties. The result was the PPI® – a sleek and aesthetically pleasing pen injector that went on to win the prestigious Red Dot Design Award for product design in 2009.
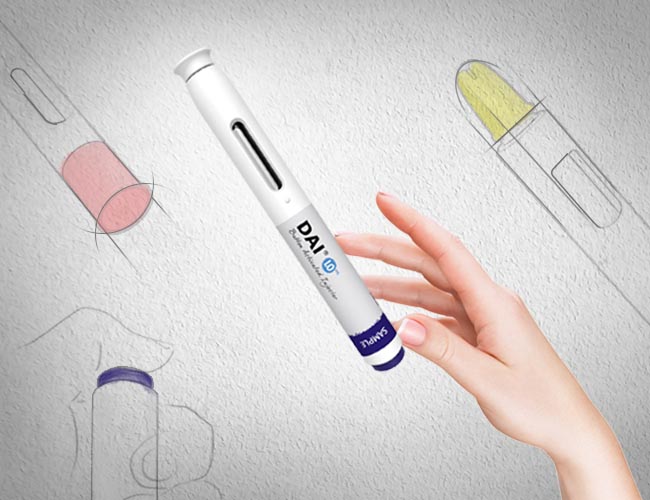
DAI®: Button activated autoinjector
2006 marked the commercial launch of one of the world’s first modern autoinjectors – SHL’s Disposable Autoinjector, more commonly known as DAI.
The first DAI was built for a multinational biopharmaceutical company headquartered in the US. It is a button-activated, 3-step disposable device for 1 mL pre-filled syringes. We were tasked to design an injection device that could facilitate an easier subcutaneous self-injection experience compared to the alternative pre-filled syringe or vial. For this reason, DAI features an ergonomic interface with two audible clicks at the beginning and end of injection, as well as an automatic delivery mechanism that successfully delivers the drug in a matter of seconds. As one of the first modern autoinjectors, DAI helped set the standard for biological self-injection treatments worldwide.
The commercial launch of DAI put SHL on the map as a recognized leader in the global autoinjector market. DAI later went on to win the 2006 Taiwan Excellence Award for its innovative design features. And while the design of modern autoinjectors has since evolved, DAI remains to be one of the most popular and sought-after autoinjectors today.
2006 marked the commercial launch of one of the world’s first modern autoinjectors – SHL’s Disposable Autoinjector, more commonly known as DAI.
The first DAI was built for a multinational biopharmaceutical company headquartered in the US. It is a button-activated, 3-step disposable device for 1 mL pre-filled syringes. We were tasked to design an injection device that could facilitate an easier subcutaneous self-injection experience compared to the alternative prefilled syringe or vial. For this reason, DAI features an ergonomic interface with two audible clicks at the beginning and end of injection, as well as an automatic delivery mechanism that successfully delivers the drug in a matter of seconds. As one of the first modern autoinjectors, DAI helped set the standard for biological self-injection treatments worldwide.
The commercial launch of DAI put SHL on the map as a recognized leader in the global autoinjector market. DAI later went on to win the 2006 Taiwan Excellence Award for its innovative design features. And while the design of modern autoinjectors has since evolved, DAI remains to be one of the most popular and sought-after autoinjectors today.
PenInject 2.25: SHL’s 1st injector project
SHL’s dedication to designing patient-centric products is evident in our very first injector, the PenInject 2.25.
Our journey into the autoinjector industry began in the mid-1990s when a Swedish pharmaceutical company tasked us to design and manufacture an easy-to-use injection device to work with its lyophilized drug. The treatment was already approved in the vial and prefilled syringe format for 2.25 mL dosing, and the injector needed to complement the original packaging to enhance the user experience.
SHL’s PenInject 2.25 was among the industry’s first to have successfully transformed a syringe-based drug product into an easy-to-use autoinjector. As a reusable, button-activated device, it offered a more intuitive alternative to the original self-injection method, including a mechanism that kept the needle hidden during the injection process. Importantly, PenInject 2.25 was given a sleek, elegant, and discreet pen-like design, affording patients an extra sense of privacy associated with their treatment. As SHL’s first injection project, PenInject 2.25 continues to represent SHL’s dedication to designing products that are centered around the patient.
SHL’s dedication to designing patient-centric products is evident in our very first injector, the PenInject 2.25.
Our journey into the autoinjector industry began in the mid-1990s when a Swedish pharmaceutical company tasked us to design and manufacture an easy-to-use injection device to work with its lyophilized treatment. The treatment was already approved in the vial and prefilled syringe format for 2.25 mL dosing, and the injector needed to complement the original packaging to enhance the user experience.
SHL’s PenInject 2.25 was among the industry’s first to have successfully transformed a syringe-based drug product into an easy-to-use autoinjector. As a reusable, button-activated device, it offered a more intuitive alternative to the original self-injection method, including a mechanism that kept the needle hidden during the injection process. Importantly, PenInject 2.25 was given a sleek, elegant, and discreet pen-like design, affording patients an extra sense of privacy associated with their treatment. As SHL’s first injection project, PenInject 2.25 continues to represent SHL’s dedication to designing products that are centered around the patient.


